Diagnosis Sp. z o.o. holds a certified quality management system according to standard EN ISO 9001 and EN ISO 13485. This company is subject to a regular, annual monitoring by TÜV Rheinland Group.The requirements of Annex IV, Article 3 of the EC directive 98/79/EC have been met.
Diagnosis Sp. z o.o. holds a certified quality management system according to standard EN ISO 9001 and EN ISO 13485. This company is subject to a regular, annual monitoring by TÜV Rheinland Group.The requirements of Annex IV, Article 3 of the EC directive 98/79/EC have been met.
Scope: Design and development, production and distribution of in-vitro diagnostic rapid tests.
Products:
- Fecal Occult Blood Self Testing Devices
- HCG Rapid Self Testing Devices
- LH Rapid Self Testing Devices
- FSH Rapid Self Testing Devices
- Blood Glucose Test Devices for Self Testing
- Blood Glucose Test Strips for Self Testing
- Drug of Abuse Urine Self Testing Devices
- Drug of Abuse Salvia Self Testing Devices
The requirements of Annex V, Article 3 of the EC directive 93/42/EEC have been met.
Scope: Production and distribution of diagnostic medical devices and of invasive sample collection devices.
Products:
- Blood Pressure Meters
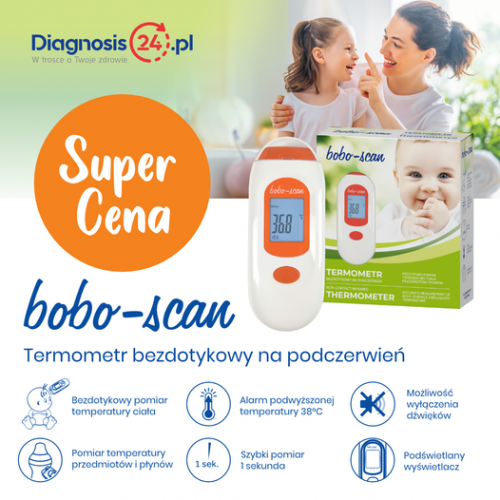
- Digital Thermometers
- Lancing Devices
The meaning of EN ISO 9001
The organization has established a quality management system that conforms to EN ISO 9001 requirements. The scope that was audited is listed at the certificate of the organization.TUV-auditors review the certified management system regularly.
The meaning of EN ISO 13485
The European Standard EN ISO 13485 defines requirements for the quality management system of organisations with respect to design, development, production, installation and customer service of medical devices as well as of design and development of the relevant services.
more: tuvdotcom.com